Quest to unravel mysteries of our gene network
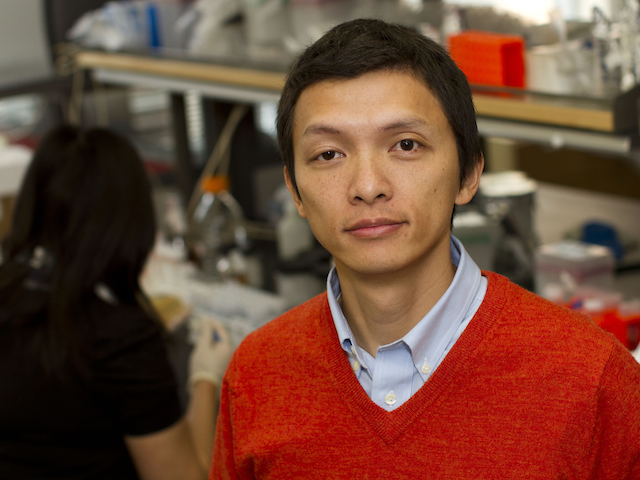
Biomedical engineer Xiao Wang is leading research to understand the fundamental nature of gene networks. Photographer: Jessica Hochreiter/ASU.
There are roughly 27,000 genes in the human body, all but a relative few of them connected through an intricate and complex network that plays a dominant role in shaping our physiological structure and functions.
Part of the complexity involves the varying and diverse functions and interplay of the genes themselves.
Some genes are activators. They trigger actions by other genes. Some genes are inhibitors. They stop other genes from acting in particular ways, for better or worse.
Some genes are both activators and inhibitors, and do different things under different conditions.
A particular action by one gene can set off a kind of chain reaction that reverberates throughout parts of the gene network and affects the overall genetic and cellular functioning.
Arizona State University biomedical engineer Xiao Wang is leading research to understand the fundamental nature of gene networks.
He wants to produce a systematic and quantitative picture to explain how and why these networks behave the way they do, and pinpoint what guides them in their “decision-making” at the cellular level.
That involves identifying gene network motifs, or patterns, in the characteristics and behavior of networks – revealing both how the genes function on their own and as part of interrelated configurations, explained Wang, an assistant professor in the School of Biological and Health Systems Engineering, one of ASU’s Ira A. Fulton Schools of Engineering.
The novel techniques Wang’s research team will apply in pursuit of new knowledge about gene networks have helped bring a grant of more than $1.5 million from the National Institute of General Medical Sciences, one of the National Institutes of Health.
“We have developed advanced experimental and computational tools to construct and study gene networks,” he said. “ We are going to combine these tools with a new microfluidics platform to gain a better understanding of the role of gene network configurations in cellular decision-making.”
The expertise in microfluidics will be provided by Wang’s lead partner in the project, Jeff Hasty, a professor of microbiology and bioengineering at the University of California, San Diego, and a principal investigator in the university’s Biodynamics Laboratory.
Wang also is getting research assistance from ASU biomedical engineering doctoral students David Menn and Fuqing Wu.
They will engineer gene networks in yeast and E. coli bacteria, which provide environments that allow the networks to grow relatively rapidly and in which gene expression can be easily observed.
The idea is for the observations to yield new insights into the basic mechanisms underlying the development of the gene network motifs that determine the functioning – and malfunctioning – of the body’s cells.
Wang said such findings could provide knowledge for extending our capabilities to more precisely target delivery of therapeutic drugs to parts of the body, to reprogram cell behavior for providing more effective implementation of regenerative medicine techniques, and to engineer synthetic biological systems that would arrest development of diseases.
The result could be a font of knowledge that would also provide a springboard to progress in vaccine development, cancer treatment, prevention of infectious diseases and molecular prosthesis.
Media Contact
Joe Kullman, [email protected]
480-965-8122
Ira A. Fulton Schools of Engineering



































