Using AI to battle Alzheimer’s
Fulton Schools researchers and Banner Health medical imaging expert team up to take steps toward more effective treatments for debilitating disease

It’s estimated that more than six million Americans — and about 24 million people worldwide — are living with the degenerative brain disease called Alzheimer’s, a progressive mental deterioration that is the fifth leading cause of death in the United States among people who are 65 and older.
The Alzheimer’s Association and other sources report that medical experts estimate the number of people with this form of dementia more than doubling within the next three decades.
That reality is leading to more research aimed at deepening knowledge about the causes and progression of the disease to help in developing effective treatments.
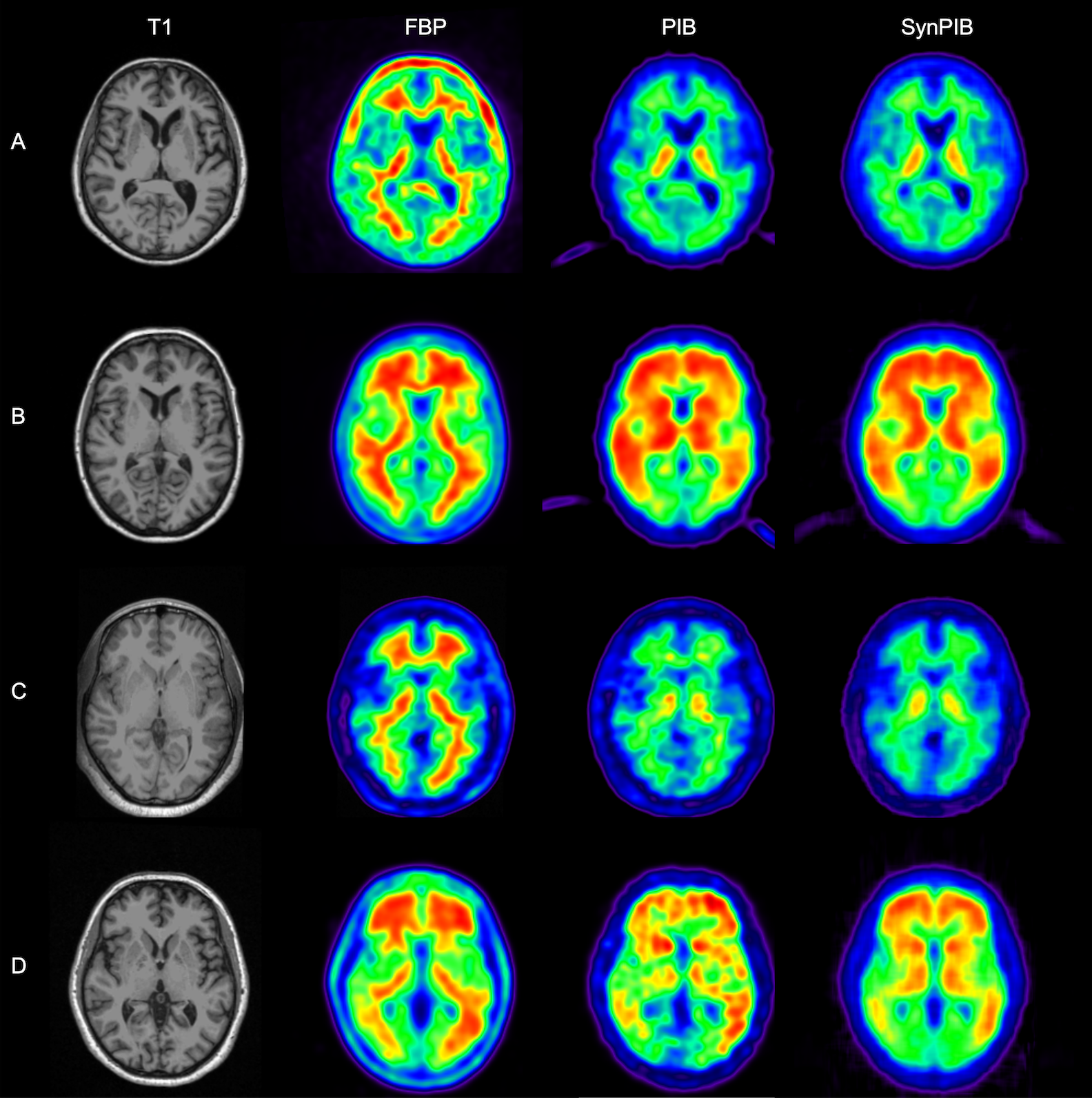
This composite image shows a visual comparison of actual positron emission tomography, or PET, images of human brains and synthetic PET brain images generated using a network built by a team of researchers from Arizona State University and the Banner Alzheimer’s Institute. These results are important to the team’s studies aimed at improving the detection and diagnosis of Alzheimer’s disease. In this figure, amyloid PET data from two commonly used the tracers florbetapir, or FBP, and Pittsburgh Compound B, or PiB, are shown (in the second and third columns) for the same set of patients. This shows obvious differences visually between images from the two tracers. The technique developed by the research team was able to generate synthetic PiB images (in the last column) based on FBP images (in the second column), producing much improved visual similarity between the synthetic PiB images and real PiB images (in the third column). Image courtesy of Yi Su’s Banner Alzheimer’s Institute research team
One essential key to success is finding ways to more accurately detect the early indicators of the disease, for example, through neuroimaging — generating images of the brain — as well as ways to more clearly visualize its physiological signs.
Advances in neuroimaging and related medical technologies would more closely reveal the specific conditions of individual patients, providing a foundation for effective analysis on which to base diagnoses and treatment options.
Among those taking on the challenge are researchers Teresa Wu, Yi Su and Jay Shah. Wu is a professor and Shah is a graduate research associate in the School of Computing and Augmented Intelligence, one of the seven Ira A. Fulton Schools of Engineering at Arizona State University. Su is the director of computational image analysis at the Banner Alzheimer’s Institute, or BAI, in Phoenix, a part of Banner Health.
Wu is also co-director of ASU-Mayo Center for Innovative Imaging and Su is one of the center’s affiliate faculty members.

Banner Alzheimer’s Institute Senior Scientist Yi Su directs the Computational Image Analysis Lab and co-leads the Biomarker Core of the Arizona Alzheimer’s Disease Research Center. His research focuses on the development and application of novel imaging techniques to study brain function and pathology, especially in cases of Alzheimer’s disease and related dementia. Photograph courtesy of Yi Su
Wu and Su are the co-senior authors and Shah is the lead author of a recently published paper in Alzheimer’s & Dementia, the journal of the Alzheimer’s Association, “Deep residual inception encoder-decoder network for amyloid PET harmonization.”
The research paper describes the intricate details of leveraging deep learning techniques and amyloid imaging with the use of positron emission tomography, or PET, tracers to provide a more accurate interpretation and better quantitative analysis as a basis for Alzheimer’s detection and diagnostics.
Amyloid plaques, as described by news-medical.net, are aggregates of abnormally configured proteins that form in spaces between nerve cells. The proteins are thought to play a central role in the development of Alzheimer’s disease. The amyloid plaques first develop in the areas of the brain associated with memory and other cognitive functions.
The brain contains tens of billions of neurons that process and transmit information by electrical and chemical signals, sending messages between different parts of the brain, and from the brain to muscles and organs. Alzheimer’s disease disrupts communications between neurons, causing loss of body function and cell death.
In their work, Wu, Su, Shah and others on their research teams from ASU and BAI, are exploring a more efficient way to analyze images produced by amyloid PET tracers, the in vivo technology used to quantify beta-amyloid deposition in the brain, which is a precursor for Alzheimer’s disease.
Multiple PET tracers are used in different research studies, and the differences between them pose a challenge for developing a consensus on accurate quantitative comparisons for Alzheimer’s diagnosis, the researchers say. So, their work is instead aimed at “harmonizing” these PET tracers through deep learning algorithms that can be used by medical experts to quantify amyloid depositions more effectively.
“By harmonizing, we are talking about making the PET images that are obtained by using different types of tracers look more similar, and also making the quantitative measures obtained from these images more comparable,” Wu says. “The goal is to allow the different amyloid PET tracers to be used interchangeably after applying our harmonization technique, so that clinicians and researchers can make the same interpretation, no matter which tracer is used.’’
The researchers have filed for a provisional patent for the harmonization technique.
“I think this work will accelerate research so that people can better leverage the data that have already been collected or from ongoing studies using different PET tracers,” says Su, whose expertise includes neuroimaging and Alzheimer’s disease.
“It will help us better understand how Alzheimer’s disease is developed, how it progresses over time, and enable data-driven approaches that facilitate Alzheimer’s disease research,” he says. “The ultimate goal is to develop effective treatments that might slow down disease progression, prevent or even cure the disease.”

Fulton Schools Graduate Research Assistant and computer scientist Jay Shah specializes in artificial intelligence and medical imaging. In addition to work in those fields, he also does research in computer vision and deep learning. Photograph courtesy of Jay Shah
The project combines Wu’s expertise in health informatics with Su’s experience in medical imaging.
Shah, a computer scientist, is adding to the mix his knowledge of machine learning and deep learning techniques, both of which intersect with artificial intelligence concepts and technologies.
“I want to improve on the modeling used to help make advances in clinical research using the combined capabilities of both machine learning and deep learning,” Shah says.
By doing that, the researchers hope to take steps toward expanding methods they have specifically designed to reveal the signs and progression of Alzheimer’s disease.
The project seems likely to lead to other patents for new techniques and methodologies the team is developing, as well as potential commercialization of the specific tools the researchers might create to perform those methods and techniques.
Progress on that front would open possibilities for support of their work from the federal Small Business Innovation Research and Small Business Technology Transfer programs, Wu says.
The research currently has funding from the Arizona Department of Health Services and from several National Institutes of Health, or NIH, grants. With advances toward their goal, Wu, Su and Shah hope to attract additional NIH support.
Wu emphasizes that whatever transpires on the commercialization and funding fronts, she and her colleagues want to ensure that the tools and knowledge they produce will be made available for use in further studies by medical researchers and to educate students.
“We want to put the results of what we are discovering into the hands of experts in many areas of science and engineering research and development.” Wu says. “That’s what will be needed to win the battle against Alzheimer’s.”


































