ASU rapidly engineers solution for medical staff battling COVID-19
Above: A team comprised of ASU Regents Professor Paul Westerhoff, fellow faculty and students have rapidly generated a practical solution for decontaminating medical face masks using ultraviolet light. Photo courtesy of Paul Westerhoff
On the third Saturday of March, only a few days after Arizona State University directed its community to work remotely in response to the COVID-19 pandemic, Paul Westerhoff received an email from a fellow faculty member in the Ira A. Fulton Schools of Engineering.
He learned that a doctor at a major hospital chain in Phoenix was seeking help with an urgent problem. The novel coronavirus had triggered a global surge in demand for personal protective equipment, or PPE, and his hospital was running out of face masks. Westerhoff contacted the doctor that same day.
“He told me that health care staff were worried about sending their masks off site for disinfection and getting other people’s masks in return,” Westerhoff said. “It’s potentially a life and death issue in the context of viruses because once an N95 mask is fit to someone’s face, it may not form a proper seal on anyone else’s face.”

Paul Westerhoff
The physician wanted a way for hospital staff to sanitize masks themselves, so Westerhoff shared the idea of creating a device for on-site disinfection using germicidal ultraviolet, or UV-C, light.
As a Regents Professor in the School of Sustainable Engineering and the Built Environment, one of the six Fulton Schools at ASU, Westerhoff already leads research on the application of ultraviolet light to decontaminate water. He also works with ASU School of Molecular Sciences Professor Pierre Herckes on a project investigating aspects of PPE use in semiconductor fabrication clean rooms. Consequently, the scientific background to solve the hospital’s problem seemed firmly in place.
“The doctor said using UV sounded great,” Westerhoff said. “In fact, he dropped off masks at my house the next morning. Then things started moving very quickly. Within four days, our team had conducted several experiments and assembled a successful prototype device.”
Within only a few more days, Westerhoff had applied for a $150,000 National Science Foundation Rapid Response Research (RAPID) grant to help fund the effort, and it was subsequently approved. By the beginning of April, his team had a fully developed device ready for deployment. As explained in the operator’s manual they wrote, it can simultaneously disinfect 16 N95 masks within two minutes.
This successful effort is a testament to the scientific knowledge, technical skill and innovative mindset of the ASU engineering community. It’s also a lesson in lateral thinking because meaningful solutions should not spark other problems.
Design around delivering a distinct dose
Westerhoff collaborated with Herckes to organize a team of five graduate students to conduct this speedy research and development at ASU’s Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment, which operates in partnership with Rice University, the University of Texas at El Paso and Yale University.
Work already in progress at the center confirmed that ultraviolet light, specifically UV-C light with wavelengths between 250 and 265 nanometers, offers sufficient energy to destroy pathogens. It does so by severing the genetic code (DNA and RNA) of microbes and compromising their proteins. But Westerhoff’s team, and the scientific community as a whole, did not know whether a dose of UV-C sufficient to neutralize the novel coronavirus would also damage the polymers comprising the N95 masks and render them ineffective for future use. Finding that answer became a central goal of the project.
UV-C dosage is measured in joules per square centimeter (J/cm2). Joules are the standard units of energy in the scientific world, but they are an esoteric concept for most people.
As a real-world comparison, the typical American household uses 30 kilowatt-hours of electricity in a given day, which equates to more than 100 million joules. This juxtaposition makes joules sound underwhelming, but the relevant area for UV-C measurements is just a single square centimeter.

Zhe Zhao
Additionally, it is the very short wavelengths of ultraviolet light that make it particularly lethal to bacteria and viruses. A little can do a lot.
“Plenty of existing literature confirms that just one joule per square centimeter (1 J/cm2) of ultraviolet light can disinfect viruses, so we designed our device around delivering that dose,” says Zhe Zhao, a first-year doctoral student of environmental engineering in Westerhoff’s lab group at ASU, and a member of the project team.
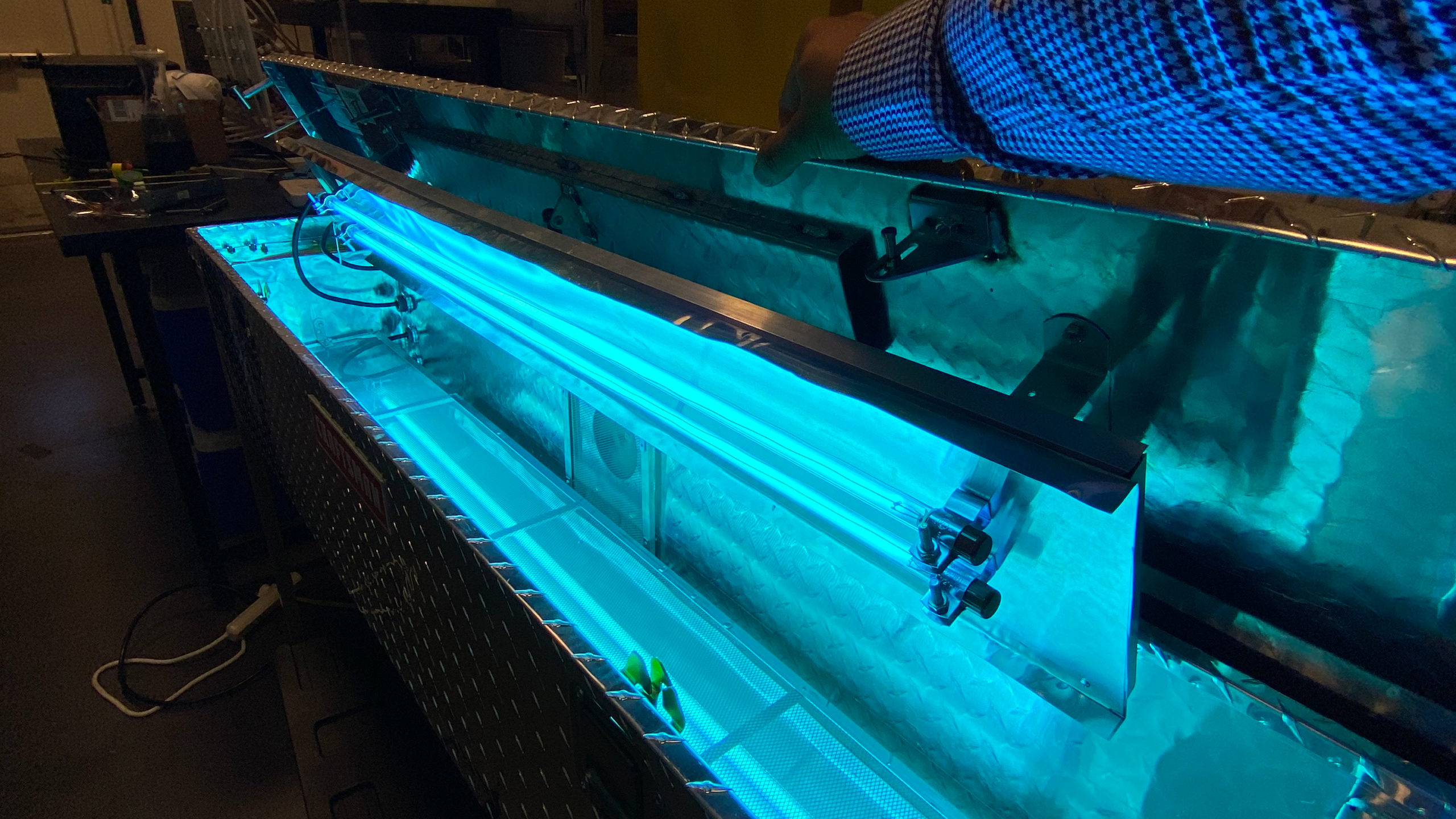
The device they rapidly fabricated resembles a large metal toolbox, similar to those commonly seen in the beds of pickup trucks. Four 120-watt ultraviolet lamps are mounted within it, each about four feet long and less than an inch in diameter. Between them, a rack positions the face masks to absorb the disinfecting UV-C. The result could be likened to a large barbecue grill.
During development, “we used a radiometer to measure the light intensity at various locations within the device,” Zhao says. “Once we had determined the spatial arrangement of intensities, we could calculate the exposure time necessary to achieve the required dose.”
With all of the components configured to irradiate contaminated masks, the key question remained: Is the delivery of sanitizing UV light damaging the mask materials? There is no point in purifying PPE from pathogens if the equipment emerges unsuitable for its purpose.
Assessing impact across the lifetime of use
The team used equipment in Herckes’ laboratory to determine the impact of their device on the structure and performance of the masks. They created aerosols of nanoparticles the same size as coronavirus virions — the “crowned” spheres displayed in countless media stories — and attempted to send them through the masks.

Mariana Lanzarini-Lopes
“The process used a small tube and a nebulizer, and it sent the nanoparticles at a sample of mask material,” says Mariana Lanzarini-Lopes, a member of Westerhoff’s team who graduated from ASU in May with a doctorate in environmental engineering. “We then scanned the sample to determine its removal efficiency or the ability to capture particulates. By doing so both prior to irradiation and afterward, we could assess the impact of the UV disinfection on the functional integrity of masks.”
The team tested the efficacy of the masks before and after administering not only the recommended single joule dose of UV, but also using a 10 J/cm2 dose. The much larger figure reflected the architecture of their decontamination device, the shape of N95 masks and the protocols for medical PPE use.
“We looked at the distribution of radiation in our box, and all parts of a mask receive at least the minimum one joule dose,” Lanzarini-Lopes says. “But the sections of a mask that are closer to the lamps may get two or three joules during exposure. Knowing that the recommended life of any disposable PPE is no more than two or three uses, the 10-joule test verified the impact of the most radiation that would ever be applied to a single mask.”
Their results were an unqualified success. Neither the single-joule irradiation nor the 10-joule irradiation generated any statistically significant changes to mask material structure or performance when compared with new, untreated mask material. This was particularly important when it came to testing the impact of UV-C exposure on the hydrophobicity, or low wettability, of the mask material.
The character of N95 filters that repels water is directly linked to their effectiveness in capturing virions by electrostatic charge. In fact, masks don’t actually represent a physical barrier to virus particles, which are much smaller than the gaps or pores in the filter medium. N95 masks function as well as they do because the virus “sticks” to the mask material. Fortunately, the UV irradiation that Westerhoff’s team used to sanitize masks does not compromise this vital particle-capturing characteristic.
“Yet through this research, we discovered that science knows very little about how the nuances of different particles influence the ability of masks to remove them,” Westerhoff says. “So, we have an opportunity now to investigate particles of different sizes and how they interact with different surfaces in order to understand how PPE can be even more effective.”
Reevaluating current practice and expanding reuse
According to Westerhoff, the team’s decontamination device may not ultimately represent an ideal tool for hospitals. He says a typical facility uses more than 5,000 N95 face masks each week, and that volume negates the utility of their innovation, even with its remarkably quick disinfection process.
“Instead, we have a real opportunity to support emergency response units,” he says. “Fire and ambulance crews respond to events in small groups, and then return to their stations for long intervals. The nature of their work lends itself to convenient, on-site mask disinfection in the numbers that our device can support.”
With this said, Westerhoff does not anticipate starting a company to commercialize this innovation. Instead, he feels inspired to offer testing and informed advice to help guide organizations that already operate as part of this industry.
More broadly, this experience represents a warning for society to evaluate our use of PPE from the standpoint of environmental impact and long-term sustainability. Westerhoff says the United States alone uses 1.5 billion N95 masks each year.
“They are designed to be used once and then thrown away,” he says. “And they’re treated as biological waste, which means they are chemically treated and then go into a landfill. Right now, it’s a very inefficient practice, so we have the ability to facilitate greater reuse and dramatically reduce waste.”



































