ASU startup pioneers breakthroughs in cell therapy
ImmunoShield Therapeutics drives innovation in biotechnology

Members of the 2024 BIO International Convention delegation with Phoenix Mayor Kate Gallego featuring ImmunoShield Therapeutic founders Jessica Weaver, Oren Snir and Matthew Becker. Image courtesy of Jessica Weaver
Regenerative medicine harnesses the body’s inherent ability to heal itself by using cells to repair or replace damaged tissues and organs. The field is full of new possibilities for disease treatment.
Jessica Weaver, an assistant professor of biomedical engineering in the School of Biological and Health Systems Engineering, part of the Ira A. Fulton Schools of Engineering at Arizona State University, is developing a new technology in the field through her startup company, ImmunoShield Therapeutics, to improve health care outcomes.
Weaver is working with co-founder Oren Snir and the company’s chief technology officer Matthew Becker, a postdoctoral research scholar in the School of Biological and Health Systems Engineering, to create a new device for hydrogel cell encapsulation, a promising subset of regenerative medicine.
Challenges in hydrogel microcapsule delivery
Transplanted cells are commonly used in regenerative medicine. Hydrogel cell encapsulation, which involves encasing cells in a water-based gel material, can make cell therapies safer by reducing the need for the long-term use of immune system-suppressing drugs after the cells have been transplanted from a donor.
“Cell therapies, particularly for conditions like Type 1 diabetes, address the root cause of the disease,” Weaver says. “In Type 1 diabetes, the body destroys insulin-producing cells, leaving patients unable to produce insulin. By replacing these cells, we can potentially eliminate the need for insulin injections, aiming for a functional cure.”
Over the past 50 years, there have been many attempts at encapsulating cell therapies for Type 1 diabetes in biomaterials. However, transitioning from preclinical models to clinical applications in humans has posed a significant challenge: ensuring adequate oxygen transport to the encapsulated replacement cells.
The experimental methods for cell therapies for Type 1 diabetes stem from the inherent complexities of effectively delivering hydrogel microcapsules in a clinical setting. Hydrogel microcapsules, typically 300 to 1,000 micrometers in diameter, encapsulate one to three islets per capsule. Islets are small clusters that contain insulin-producing cells.
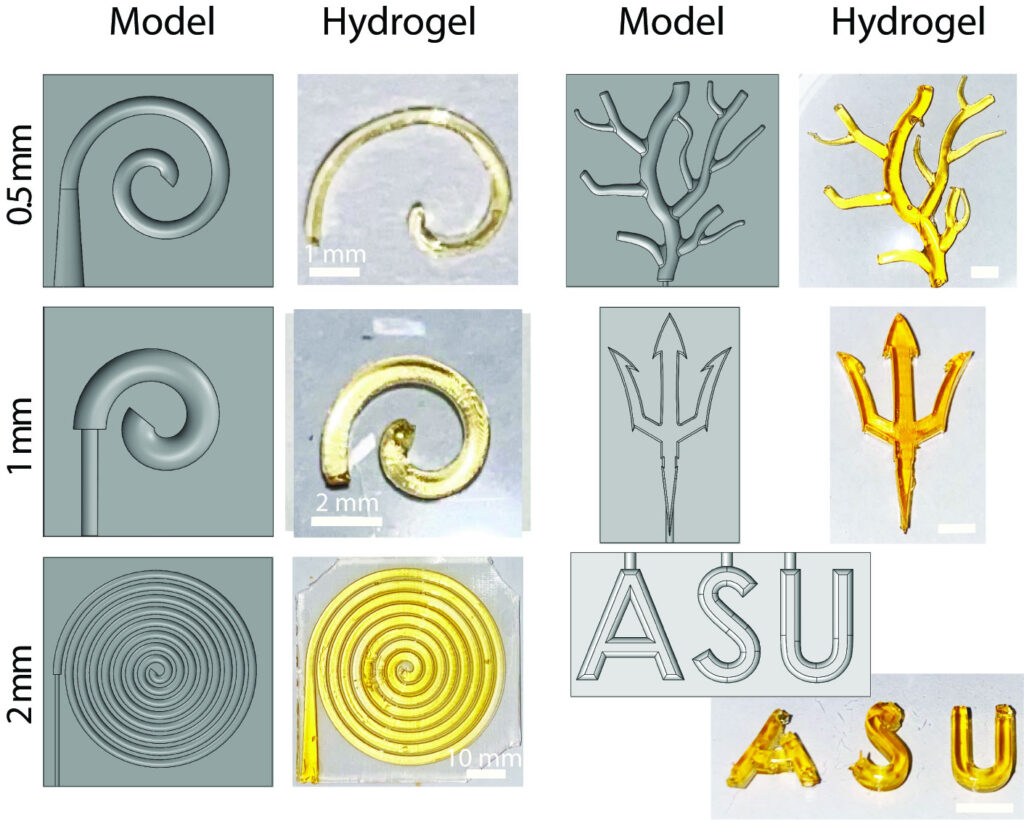
Models developed for hydrogel injection molding in biomedical applications. This innovative research, led by Jessica Weaver and Matthew Becker, showcases the potential of hydrogels in medical science. Image courtesy of Jessica Weaver
Due to the volume requirements, these capsules are generally delivered in the abdomen However, inserting the capsules in the abdomen makes retrieval challenging if complications occur.
In cases of adverse events or cells not functioning properly after being transplanted, also known as graft failure, retrieving the capsules can be extremely difficult. This poses a significant risk, as it may not be possible to remove the capsules quickly and safely in the event of complications.
The survival and function of the encapsulated cells are heavily dependent on an adequate supply of oxygen from the surrounding environment into the hydrogel encapsulation where the cells are housed. Insufficient oxygen, can lead to cell death and impaired functionality, undermining the therapeutic effectiveness of the encapsulated cells.
Over time, capsules can also develop fibrosis, which impedes the function of the cells inside the capsules. Fibrosis blocks the exchange of essential nutrients and waste products and complicates the retrieval process further by causing the capsules to adhere more firmly to surrounding tissue.
Firm adherence to abdominal organs and tissues can cause complications such as inflammation or infection and impact the overall success of cell therapy.
Enhancing the design of these capsules to facilitate better oxygen transport, reduce fibrosis and prevent adhesion to tissues has the potential to significantly improve cell therapy outcomes.
Cell macroencapsulation as an alternative
At the heart of ImmunoShield’s endeavor is the macroencapsulation approach, a technique that involves placing a large number of cells into a single hydrogel device.
The approach allows for the safe retrieval of the device from a patient if therapy is unsuccessful or causes unforeseen problems.
Despite macroencapsulation’s advantages, oxygen supply is still an issue.
“The problem with encapsulation, particularly macroencapsulation, is that larger devices containing cells often struggle with oxygen transport,” Weaver says. “These devices have difficulty ensuring that the cells inside receive enough oxygen.”
ImmunoShield aims to ensure adequate oxygen delivery by creating complex 3D geometries for their encapsulation devices. These complex geometries have higher surface area-to-volume ratios than others, providing more oxygen to the encapsulated cells.
For cost-effective manufacturing, ImmunoShield has developed a technique called hydrogel injection molding, which results in highly efficient and scalable production. This process could reduce the cost of cell therapies and increase patient accessibility.
Weaver also sees potential for applications beyond treating Type 1 diabetes.
“Our expertise is specifically in Type 1 diabetes and developing cell therapies for that application, but we’re not limited to marketing it in that space,” she says. “The application of the automated injection molding system is much broader.”
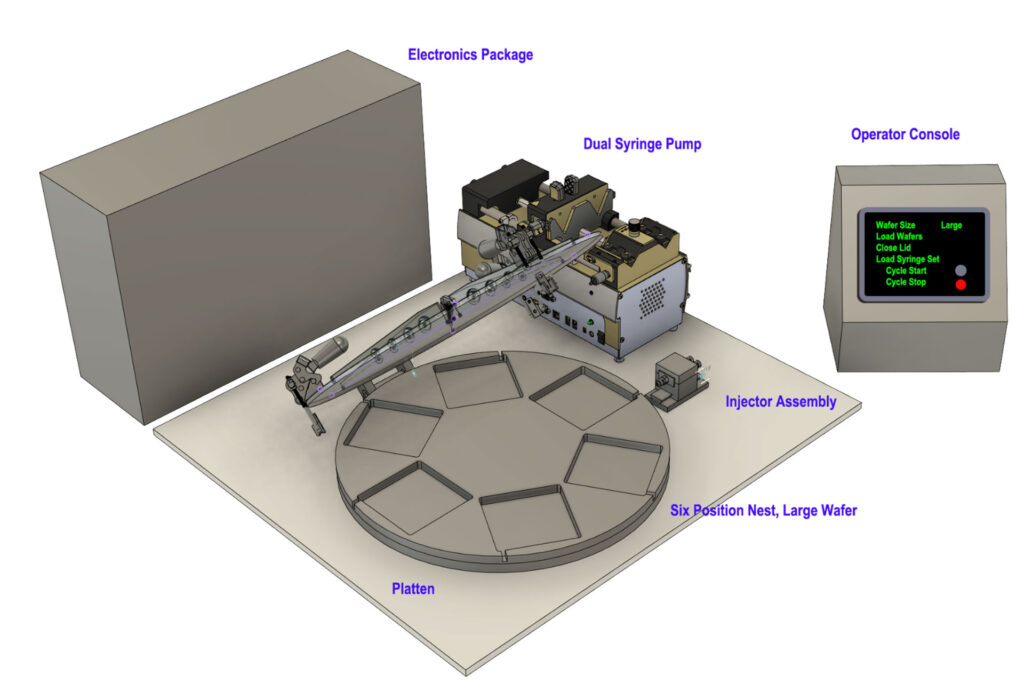
A 3D rendering of ImmunoShield Therapeutic’s automated hydrogel injection molding system. Image courtesy of Jessica Weaver
A leader among Phoenix biotechnology
Weaver and Becker recently attended the BIO International Convention in Phoenix and presented ImmunoShield’s technologies. The convention brings together experts and businesses in one of the largest markets in the U.S. for biotechnology companies.
“The convention attendees valued my expertise and my commitment to the company and thought I would bring some much-needed time commitment to help commercialize our technologies and products,“ Becker says. “We’re at a stage where we’re ready to start raising serious capital and commercializing our technologies.”
Weaver has noticed that the city of Phoenix is actively investing in biotechnology industry, fueling innovation and expanding job opportunities for those interested in the field.
“Phoenix wants to be the next hub for biotech, and I believe we’re in the top five metro areas in the United States,” Weaver says. “This aspiration underscores the city’s commitment to becoming a leader in the biotech industry.”