
Discovering pathways for neural development
ASU researcher Madeline Andrews is investigating how leukemia inhibitory factor affects brain cell growth
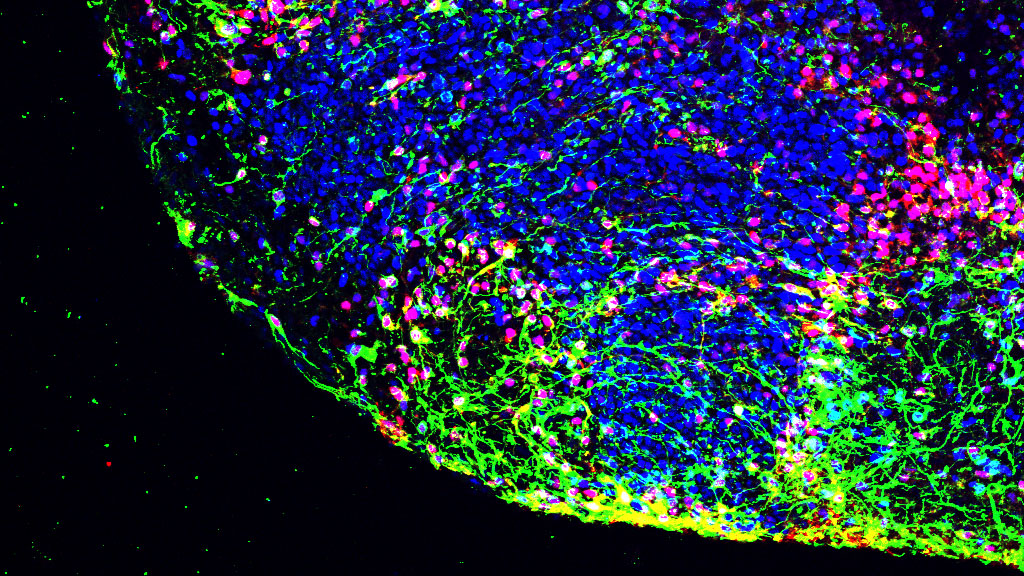
A microscopic view of radial glial cells in the brain. Green light labels the structure of radial glial fibers. The red light marks the outer radial glia nuclei by tagging the protein HOPX, and blue light labels DNA, which identifies the nucleus of each cell. Arizona State University researchers are identifying the role of leukemia inhibitory factor signaling pathways in brain growth. Photo courtesy of Madeline Andrews
Radial glial cells play a pivotal role in the body by providing structural support and serving as the stem cells of the nervous system. These cells are essential for the development of a healthy cerebral cortex due to their function of shaping cellular differentiation, a process in which genetic “blank canvases” gain distinct biological functions.
To understand how brain formation sometimes goes awry, researchers are studying how radial glial cells develop. Despite their crucial role in the body, the mechanisms regulating outer radial glia, an expanded population of neural stem cells in the human brain, and their differentiation are unclear.
Madeline Andrews, an assistant professor of biomedical engineering in the School of Biological and Health Systems Engineering, part of the Ira A. Fulton Schools of Engineering at Arizona State University, is uncovering the role of leukemia inhibitory factor, or LIF, signaling pathways on the production of neurons in the brain that regulate cognition and executive function.
Andrews uses her expertise in the molecular processes that guide the nervous system’s development to determine factors that contribute to neurodevelopmental disorders.
Investigating at the cellular level
In a recent publication in the scientific journal Cell Stem Cell, Andrews establishes that LIF activity regulates how outer radial cells differentiate. Radial glial cells are known to develop into excitatory neurons. This study was the first to identify that LIF enables the outer radial glial to also develop into inhibitory interneurons, which are particular types of neurons that act as messengers sending signals between other neurons. Excitatory and inhibitory neurons activate or deactivate, respectively, signals that influence cell function and brain activity.
Arnold Kriegstein, a professor of neurology at the University of California, San Francisco Weill Institute for Neurosciences and collaborator to the project, says the LIF signaling pathway may have a greater impact on neurological function than previously theorized.
“Outer radial glial cells are heavily involved in the developing human brain and are credited with the production of excitatory nerve cells,” Kriegstein says. “These findings suggest a potential role for LIF signaling in neurodevelopmental disorders such as autism and epilepsy where the balance between inhibition and excitation is disrupted.”
Andrews, Kriegstein and their teams began by looking at how cells in the brain develop in this particular region when LIF signaling was manipulated.
When they checked their tissue cultures, the researchers observed an increase in cell replication among burgeoning neurons and, to their surprise, an increase in inhibitory interneurons.
To confirm the source of the inhibitory interneurons, they isolated the outer radial glial cells to allow for spontaneous differentiation and confirmed their previous result. When testing the genetic composition of the new neurons, the researchers determined they resembled cells in the caudal ganglionic eminence, a different region of the brain where inhibitory interneurons are also produced. This affirmed their observation that LIF signaling can influence the composition of the developing brain’s neurons.
Implications changing knowledge of brain development
Andrews says understanding signaling pathways is pivotal for researchers to interpret the impact of unbalanced cell ratios in neurological disease.
“These findings have implications for our understanding of differences in how the human brain develops compared to other species,” Andrews says. “By better defining the variety of cell types present under certain health conditions, we can identify dysfunction in neurodevelopmental disorders where there is known dysregulation of excitatory and inhibitory balance.”
While the research sheds light on a previously unknown regulation of human brain tissue, the potential sources of LIF signaling are still unknown. The impact of downstream consequences of LIF-imbalanced interneurons is also unclear, though there are significant implications for altered neuronal excitability.
“By improving human neural model systems through novel discoveries and tissue engineering strategies,” Andrews says, “we can more accurately identify the molecular drivers of neurological disorders and better understand human brain health.”


































